- Description
Easier, more comprehensive microsatellite instability testing
Using as little as 2 ng of FFPE-derived DNA, the new Applied Biosystems TrueMark MSI Assay has a panel of 13 microsatellite markers to test for MSI associated with Lynch syndrome-related cancers beyond colorectal cancer and studying immunotherapy. The included analysis and reporting software featuring automated calling can help you have a more efficient workflow.
What is microsatellite instability and why is it important?
Microsatellite instability (MSI) is the condition of genetic hypermutability that results from impaired DNA mismatch repair (MMR). Studies indicate that the presence of high levels of MSI (MSI-H) might be predictive of Lynch syndrome, a hereditary condition associated with increased cancer risk across a variety of tumor types, including colorectal, gastric, and endometrial cancers.
Defects in MMR also result in novel somatic mutations in unrelated loci throughout the genome. These mutations can produce immunogenic proteins that can “prime” an immune response to the foreign neoantigens in the tumor. MSI-H tumors are potentially more sensitive to immune checkpoint inhibitor treatment than microsatellite-stable (MSS) tumors, making MSI analysis across diverse tumors important for the identification of those whose growth might be slowed by immunotherapy treatments.
Easier MSI testing
The TrueMark MSI Assay is a low-input, affordable assay with an expanded 13-MSI-marker panel and includes TrueMark MSI Analysis Software. The entire workflow takes as little as four hours to determine sample status. The TrueMark MSI Assay leverages a 6-dye set to incorporate the additional markers and does not require the use of a tumor-normal match, cutting the number of wells required per sample in half and reducing sample handling burden.
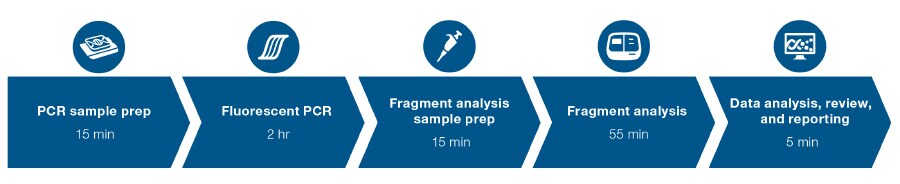
Figure 1. Enjoy a simple, rapid workflow. Go from extracted DNA to results in as little as four hours with less than one hour of hands-on time.
Features of the TrueMark MSI Assay include:
- Existing workflow compatibility—designed to run on the SeqStudio and 3500 Series genetic analyzers
- Expanded panel—13 microsatellite markers (including the Bethesda panel) to test for MSI associated with Lynch syndrome-related cancers and to study immunotherapy
- Low input—use as little as 2 ng FFPE-derived DNA
- Rapid workflow—go from extracted DNA to results in as little as four hours with less than one hour of hands-on time
- Analysis software—kit includes reporting software for automated calling of markers and reporting software
Simple MSI data analysis and reporting software
Included at no additional cost, TrueMark MSI Analysis Software is designed specifically for the TrueMark MSI Assay to enable automated calling of markers and samples, offering greater ease of analysis by reducing the manual QC, analysis, and review required to determine sample status.
Description
Easier, more comprehensive microsatellite instability testing
Using as little as 2 ng of FFPE-derived DNA, the new Applied Biosystems TrueMark MSI Assay has a panel of 13 microsatellite markers to test for MSI associated with Lynch syndrome-related cancers beyond colorectal cancer and studying immunotherapy. The included analysis and reporting software featuring automated calling can help you have a more efficient workflow.
What is microsatellite instability and why is it important?
Microsatellite instability (MSI) is the condition of genetic hypermutability that results from impaired DNA mismatch repair (MMR). Studies indicate that the presence of high levels of MSI (MSI-H) might be predictive of Lynch syndrome, a hereditary condition associated with increased cancer risk across a variety of tumor types, including colorectal, gastric, and endometrial cancers.
Defects in MMR also result in novel somatic mutations in unrelated loci throughout the genome. These mutations can produce immunogenic proteins that can “prime” an immune response to the foreign neoantigens in the tumor. MSI-H tumors are potentially more sensitive to immune checkpoint inhibitor treatment than microsatellite-stable (MSS) tumors, making MSI analysis across diverse tumors important for the identification of those whose growth might be slowed by immunotherapy treatments.
Easier MSI testing
The TrueMark MSI Assay is a low-input, affordable assay with an expanded 13-MSI-marker panel and includes TrueMark MSI Analysis Software. The entire workflow takes as little as four hours to determine sample status. The TrueMark MSI Assay leverages a 6-dye set to incorporate the additional markers and does not require the use of a tumor-normal match, cutting the number of wells required per sample in half and reducing sample handling burden.

Figure 1. Enjoy a simple, rapid workflow. Go from extracted DNA to results in as little as four hours with less than one hour of hands-on time.
Features of the TrueMark MSI Assay include:
- Existing workflow compatibility—designed to run on the SeqStudio and 3500 Series genetic analyzers
- Expanded panel—13 microsatellite markers (including the Bethesda panel) to test for MSI associated with Lynch syndrome-related cancers and to study immunotherapy
- Low input—use as little as 2 ng FFPE-derived DNA
- Rapid workflow—go from extracted DNA to results in as little as four hours with less than one hour of hands-on time
- Analysis software—kit includes reporting software for automated calling of markers and reporting software
Simple MSI data analysis and reporting software
Included at no additional cost, TrueMark MSI Analysis Software is designed specifically for the TrueMark MSI Assay to enable automated calling of markers and samples, offering greater ease of analysis by reducing the manual QC, analysis, and review required to determine sample status.


